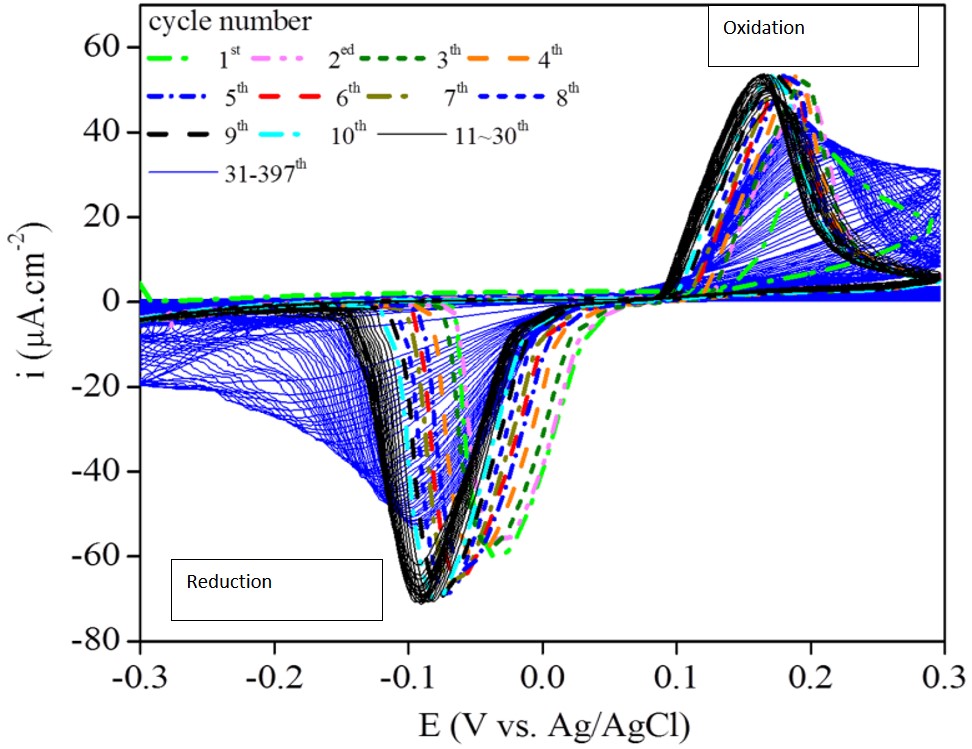
Due to unique electrical conductivity and redox properties of conducting polymers, they have been explored for active corrosion protection of metals, exploiting redox phenomena to enable the self-healing of polymer coatings. While self-sealing has been extensively studied, proving the effect of redox action has been a challenge. We have combined electrochemical techniques and in-situ atomic force microscopy (AFM) to study corrosion protection mechanism of carbon steel with polymer composite coatings containing polyaniline (PANI). The results revealed reversible oxidation and reduction of PANI with a corresponding volume change in the coating. As an example, the corrosion protection mechanism of a solvent-borne alkyd coating containing 1 wt.% PANI on carbon steel in 3 wt.% NaCl solution was investigated by cyclic voltammetry (CV) and electrochemical AFM measurements under different applied potentials. The CV curves (Figure 1) and electrochemical AFM images (Figure 2) demonstrated a reversible conversion between the oxidized emeraldine salt and reduced leuco-emeraldine base of PANI.
OCP and electrochemical impedance spectroscopy measurements during long-term exposure gave rise to an enhanced stability of corrosion protection by the composite coating, which can be attributed to redox conversion and self-healing effect of the PANI.