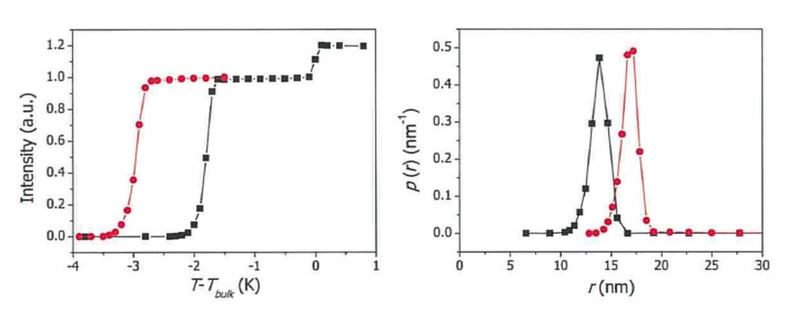
NMR spectroscopy is remarkably useful when it comes to the study of transitions between solid and liquid phases. Those two material states can easily be separated in terms of their NMR signal where the underlying reason is their different spin relaxation properties. This feature is exploited in a number of applications. One of them is NMR cryoporometry, a versatile tool in the determination of porosity. The methodology is based on depression of the melting and freezing points in pores compared to those of the bulk liquid.1 This technique can distinguish pores with diameters in the nm-mm range. Porosity and pore size distribution can be quantified but also an evaluation of the pore shape is accessible. No chemical preparation is needed except the filling of the pore structure with a suitable reference liquid like water or hexane. The porous material can be almost anything: organic or inorganic, hydrophobic or hydrophilic, with examples including ceramics, textile, minerals, carbons, waxes, concrete, wood, gels, or paper. One unique option is to study porosity in materials such as gels whose porous structure is attained in the presence of the pore-filling solvent. Namely, the pore structure in such materials is not accessible by other techniques such as gas sorption or mercury porosimetry that require empty pores.
References
1 O. V. Petrov and I. Furó, NMR cryoporometry: principles, applications and potential, Prog. Nucl. Magn. Reson. Spectrosc. 54 97-122 (2009). DOI: 10.1016/j.pnmrs.2008.06.001