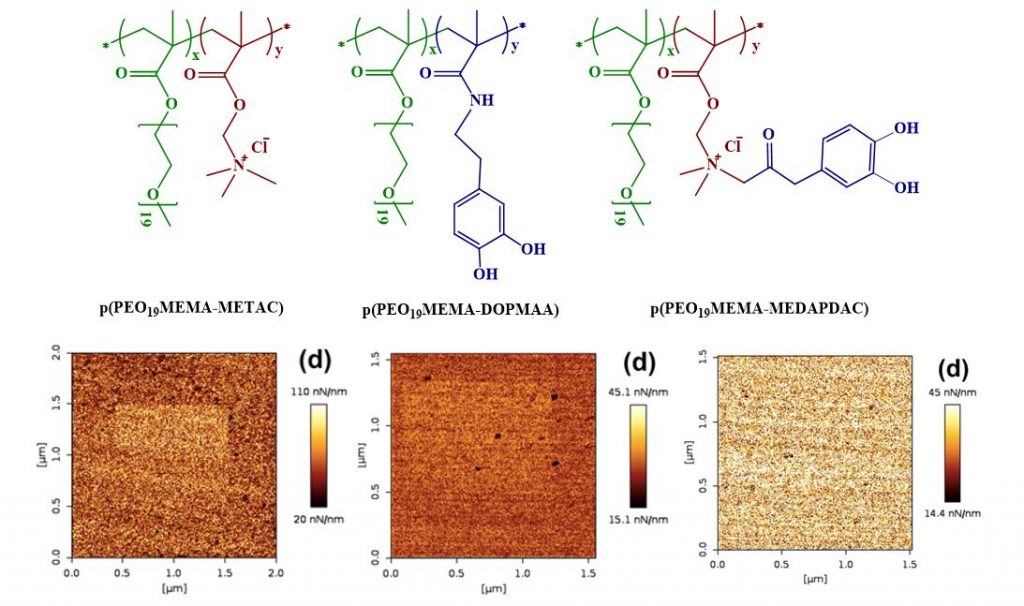
Molecular adsorption strength is not related to the adsorbed amount. For instance, polyelectrolytes with low charge density adsorb to oppositely charged surfaces in higher amount than a high charge density polyelectrolyte, whereas the adsorption strength is lower. When surfaces are modified with thin adsorbed layers to create, for example, a non-fouling surface or a well lubricated surface, it is important that the adsorption strength is high to promote a long lifetime and function of the layer. In this study we explored how different functional attachment mechanisms affect the adsorption strength by investigating molecular wear. A complication is that wear scars of sub-nanometer depth are difficult to see even with atomic force microscopy. An alternative for soft adsorbed layers on hard surfaces, is to measure the change in surface stiffness with increasing wear. An increase in stiffness is a strong indication that the adsorbed layer thickness is decreasing (due to wear), as the hard substrate will have an increasing effect on the measurement.
We compared three polymers differing only in anchoring mechanism – electrostatic (NH3+), H-bonding / dispersion interaction (catechol) or both, and found that the adsorption strength varied as NH3+ plus catechol > catechol > NH3+ anchoring when studied on silica surfaces in water.
References
Langmuir 35, 15515 (2019). DOI: 10.1021/acs.langmuir.9b01818Technique Guides