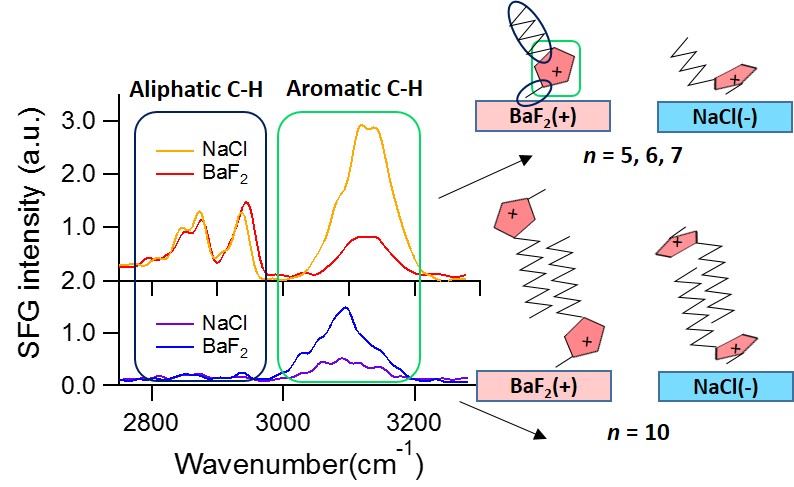
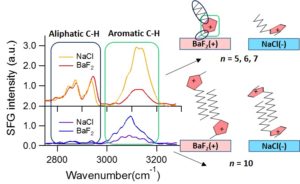
Control of the interfacial structures of ionic liquids (ILs) at charged interfaces is important for a number of their applications, including in energy storage solutions, sensors and advanced lubrication technologies utilising electric fields. In the case of the latter, there is an increasing demand for the study of non-halogenated ILs, as many fluorinated anions have been found to produce corrosive and toxic halides under tribological conditions. The interfacial structuring of a series of imidazolium ILs ([CnC1Im]) of varying alkyl chain lengths (n = 5, 6, 7, 10) with a non-halogenated borate-based anion ([BOB]), have been studied at charged interfaces using sum frequency generation (SFG) spectroscopy and neutron reflectivity (NR).
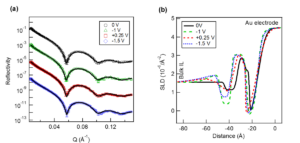
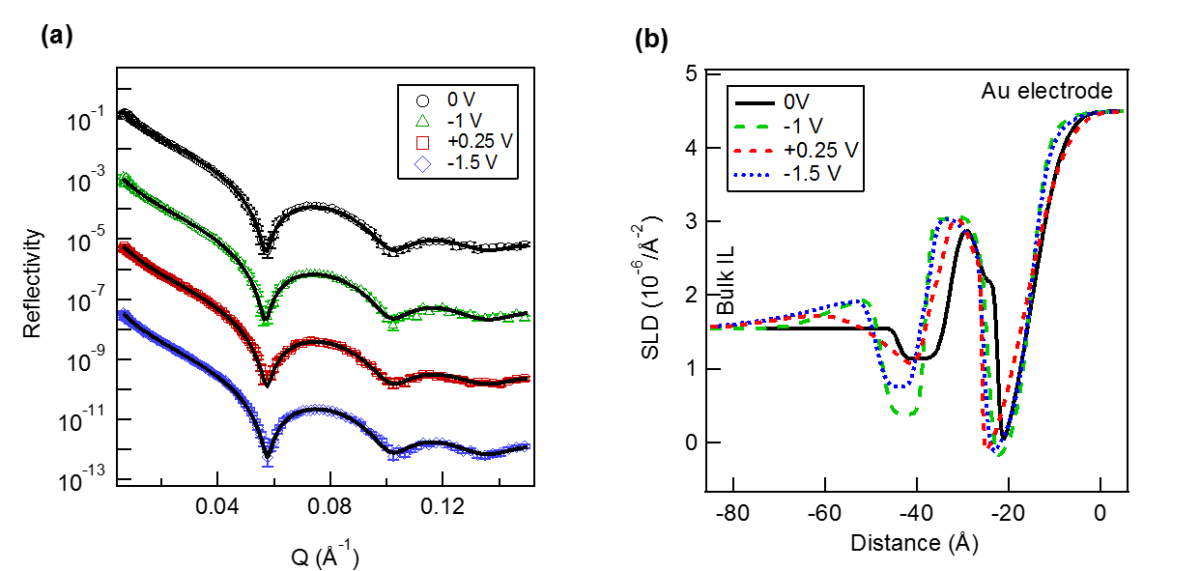
For all alkyl chain lengths, SFG spectra show that the cation imidazolium ring responds to the surface charge by modifying its orientation with respect to the charged interface. In addition, the combination of SFG spectra with electrochemical NR measurements reveals that a longer alkyl chain length (n = 10) leads to a bilayer structuring at all charged interfaces, independent of the ring orientation. These results demonstrate the tunability of IL interfacial layers through the use of surface charge, as well as effect of the cation alkyl chain length, and provide valuable insight into the charge compensation mechanisms of ILs, which are likely to be important in many of their applications.
References
Phys. Chem. Chem. Phys., 2020, 22, 8450-8460. DOI:10.1039/D0CP00360CTechnique Guides