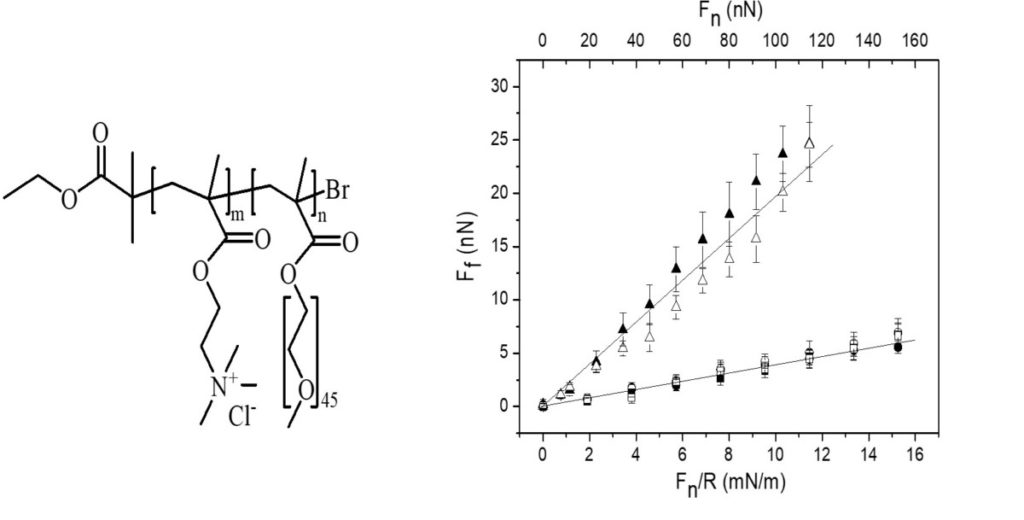
| In most technical lubrication applications, oils with suitable additives are used. Natural systems are by contrast lubricated with water-based solutions, which is also a possible strategy for technical applications at low temperature and low pressure. The basis of aqueous lubrication is the presence of a strongly bound yet easily sheared water layer, and for this reason aqueous lubricants always contain regions that are strongly hydrophilic. This strategy allows our joints to move with friction coefficients as low as 0.001 up to pressures of 250 atm. Synthetic aqueous lubricants can be designed following two criteria: i) they should bind strongly to the surface being lubricated thereby providing a high load bearing capacity, ii) it should be strongly hydrated to give a low friction force. A series of studies have been performed to explore the ability of cationic bottle-brush polymers with ethylene oxide side chains to lubricate negative charged surfaces in water. Anchoring the lubricant to the surface with the aid of electrostatic interactions has the disadvantage that the load bearing capacity decreases with increasing salt concentration. This situation can fortunately be alleviated by using non-electrostatically bound catechol groups. |
References
Phys. Chem. Chem. Phys. 19, 23677 (2017). DOI: 10.1039/C7CP03517ASoft Matter 9, 5361 (2013). DOI: 10.1039/C3SM27862J
Langmuir 24, 3336 (2008). DOI: 10.1021/la703229n