Control of the interfacial structures of ionic liquids (ILs) at charged interfaces is important for a number of their applications, including in energy storage solutions, sensors and advanced lubrication technologies utilising electric fields. For these applications, there is also an increasing demand for the synthesis of more green and sustainable (in particular non-halogenated) ILs that exhibit good miscibility in broad range of solvents. However, despite the great interest that ILs have received as electroactive agents or additives, our current understanding of the interfacial electroresponsive behaviours of IL-solvent mixtures is still lacking.
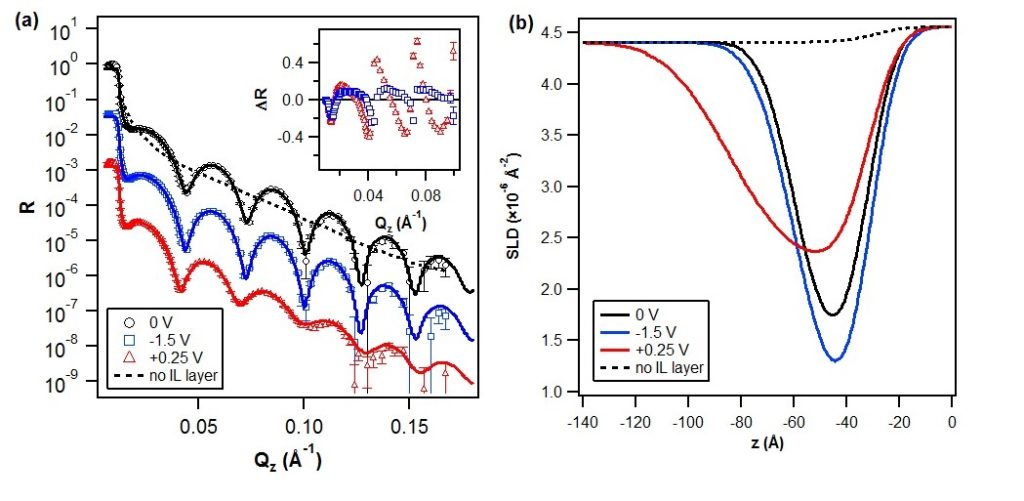
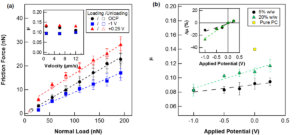
The interfacial structuring of a non-commercial phosphonium orthoborate IL ([P6,6,6,14][BMB]) at a gold electrode interface was studied using neutron reflectivity (NR). The results provide a direct characterisation of both composition and structure of the IL ions in the interfacial region, and reveal strong dependencies on the potential bias and bulk IL concentration. Previously, this particular IL has been identified as promising lubricant additive. The nanolubrication and tribotronic (i.e., frictional response to electric fields) measurements using atomic force microscopy (AFM) reveal the IL layers to provide both excellent lubrication reduction and tribotronic responsivity, whereby the friction coefficient is shown to systematically vary depending on the applied potential bias and magnitude.
References
Physical Chemistry Chemical Physics 2020, 19162-19171. DOI: 10.1039/D0CP02736GTechnique Guides