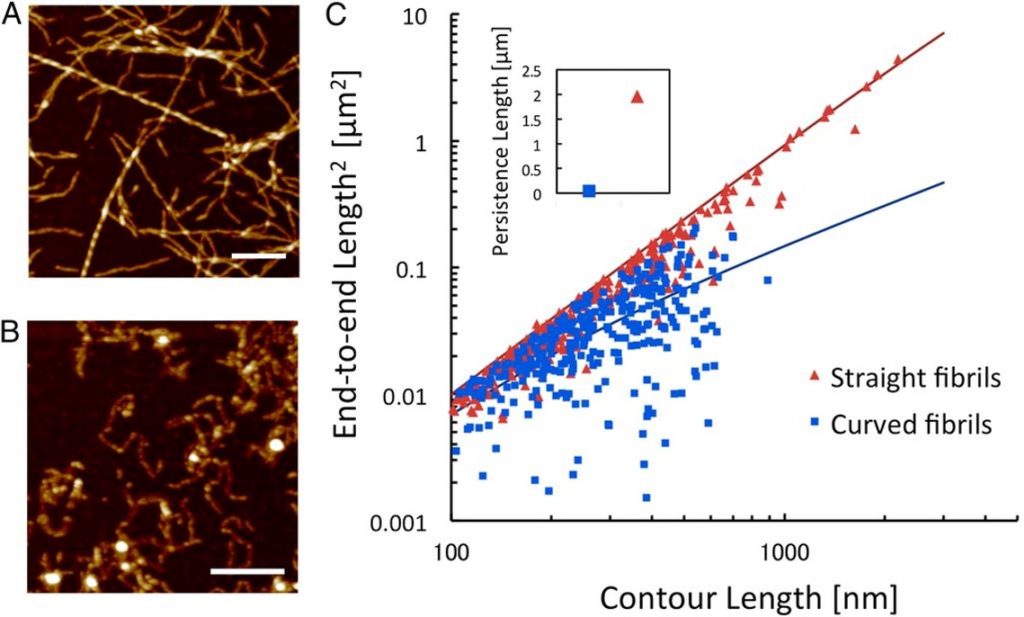
Protein nanofibrils are gaining increased interest for the design of functional bio-based materials. The nanofibrils form through self-assembly in aqueous solution from a wide range of protein raw materials. The mechanical properties of individual fibrils are comparable with silk, with elasticity in the GPa range. However, the nanoscale structure of the fibrils can alter their mechanical characteristics as well as their ability to form larger structures. We used image analysis of atomic force microscopy data to quantify the persistence length in order to compare the nanomechanical properties of two classes of nanofibrils derived from whey protein. Persistence length was calculated by comparing the end-to-end distance and the contour length of individual fibrils. This approach is based on the assumption that fibrils interact weakly with the mica support and are able to relax to 2D equilibrium.